

What is a master file FDA?įor CBER’s purposes, master files (MFs) are voluntary submissions of information to the FDA that may be used to provide confidential, detailed information to the FDA about the facilities, processes, or articles used in the manufacture, processing, packaging, or storing of one or more regulated products. The current mailing address for CDRH’s DCC and a link to CBER’s DCC’s mailing address are provided on the eCopy Program for Medical Device Submissions webpage. An MAF and all amendments should be submitted to the appropriate Center’s Document Control Center (DCC).

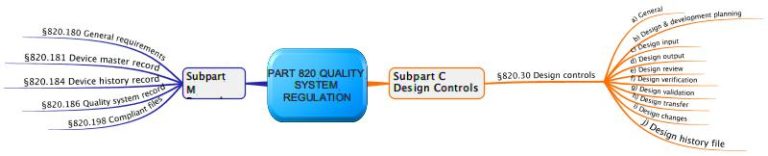
How do I submit a master file to the FDA? What should be included in a device master record?Ī DEVICE MASTER RECORD (DMR) CONTAINS ALL OF THE INFORMATION AND SPECIFICATIONS NEEDED TO PRODUCE A MEDICAL DEVICE FROM START TO FINISH, INCLUDING INSTRUCTIONS FOR ALL MANUFACTURING PROCESSES, DRAWINGS, DOCUMENTED SPECIFICATIONS AND LABELING AND PACKAGING REQUIREMENTS. THE FDA MANDATES THAT MEDICAL DEVICE COMPANIES PRODUCE A DEVICE HISTORY RECORD (DHR) THAT CONTAINS ALL DOCUMENTATION RELATED TO MANUFACTURING AND TRACKING THE DEVICE, AND DEMONSTRATES THAT THE DEVICE WAS MANUFACTURED ACCORDING TO THE INFORMATION IN THE DEVICE MASTER RECORD. Design verification and validation protocols and reports.



 0 kommentar(er)
0 kommentar(er)
